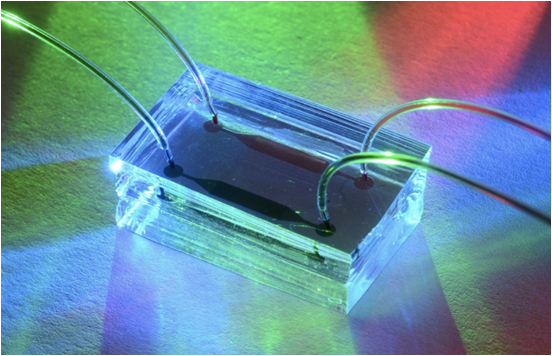
The liver and the kidney are the two most important organs involved in the metabolism of therapeutic drugs. Damage to the liver by pharmaceutical drugs, hepatotoxicity or drug-induced liver injury (DILI), is a major reason drugs are recalled from the marketplace or fail during late stages of drug development. Current technologies can fail to predict the hepatotoxicity potential while a new drug is still in its early phase of development. This can result in huge financial losses for a pharmaceutical company if the hepatotoxicity is not recognized prior entering the marketplace. More importantly, this failure to identify hepatotoxicity can result in serious patient harm or death. New technologies designed to identify hepatotoxicity early in drug development are being developed by our investigators. These technologies incorporate human liver and kidney cells to create an “organ-on-a-chip” environment to better understand key features that can lead to hepatotoxicity.
Read MoreChallenges putting primary human cells into tissue culture
Many difficult challenges arise when attempting to create an organ-on-a-chip environment that mimics the interactions of coordinated human organs but at least a few of these challenges are being addressed. By organ-on-a-chip, we mean the development of a device, which not only contains primary human cells (e.g. cells derived directly from organs like liver or kidney) but also maintains cellular function at in vivo levels. One challenge to overcome is the natural tendency of primary human cells transitioning from in vivo to in vitro to lose viability and long-term function. There are multiple approaches that begin to overcome this challenge. Another challenge has been to identify readily available and reliable sources of fresh human cells. The current approach is to acquire human liver cells that are derived from harvested livers; these livers are disaggregated and cryopreserved for storage and transportation. The cells from these rejected livers are not ideal because they are not coming from a healthy liver. Also, the cryopreservation process and the subsequent thawing of the isolated liver cells can further damage cellular membranes and compromise cellular viability and function.
Studying drug metabolism
An organ-on-a-chip device could be an excellent way to study human drug metabolism in vitro to identify the hepatotoxicity potential of a drug before giving the drug to human volunteers or patients. Drug metabolism, which predominantly occurs in the liver, is the biochemical modification of drugs into metabolites that are more easily eliminated from the body primarily by the liver or kidney. The drug metabolism process is divided into three major phases: enzymes such as cytochrome P450 oxidases introduce reactive or polar groups to drug (phase I); the modified drug is then conjugated to polar compounds utilizing transferase enzymes (phase II); and the conjugated drug may or may not be further processed before being recognized by transporters and pumped out of cells (phase III).
Why must the cells be primary human cells?
The current technologies that often fail to identify heptotoxicity in early phase drug development come from many different cellular origins including: immortalized cells from human cancers (e.g. Hep G2), cells derived from stem cells, or cells from other species, e.g. rats. Hep G2 is a perpetual cell line from a human hepatocellular cancer. Many of the normal liver cell functions are expressed in the Hep G2 cells but some critical drug metabolism features are limited or absent. In general, human cell lines lack relevant levels of phase I activity, phase II conjugation, and phase III activity. Pluripotent stem cells potentially could be coaxed into becoming differentiated hepatocytes but to date, this has not been accomplished or practically carried out. Lastly, cells from other species (e.g. rats) have been considered and used, but the pathways for chemical detoxification are all too often, species-specific. Because of the limitations in these alternative cellular sources, human primary hepatocytes present the best option for human-on-a-chip.
Improving primary hepatocyte functionality in vitro
Because human liver cells do not proliferate or grow in vitro, the best outcome is to maintain the viability of as many primary cells as possible and to promote their stable function as close to what it had been when they were in vivo. The process involves thawing the hepatocytes and adding them to the in vitro device (“seeding”) while allowing as many of the hepatocytes as possible to attach to surfaces of the device. These newly-thawed hepatocytes require a tremendous oxygen requirement during the first 24 hours of the seeding process. After seeding, the hepatocyte oxygen requirement is reduced by half but remains much greater than for most other human cells (e.g. primary kidney cells).
Primary hepatocyte functionality can be further improved by multiple 3D approaches to support long-term synthetic and metabolic in vivo-like function. Human liver contains parenchymal cells and non-parenchymal cells that have direct interactions that influence the long-term stability and cellular function of hepatocytes that are not seen in simple seeding technologies. Several 3D approaches that that have been pioneered in the Center include: entrapment of hepatocytes between two layers of collagen in a sandwich 3D configuration, which tends to promote the hepatocyte’s normal polarity, co-culturing with non-parenchymal cells such as fibroblasts and/or endothelial cells. Recently, we have published papers showing that we can maintain function in a 4-cell (hepatocytes, endothelial cells, stellate cells and Kupffer cells) liver microdevice for 28 days in culture, the preferred time period for evaluating drug toxicity in animal studies.
Microscale cell culture analog
Variations of these 3D approaches have been used by our investigators in vitro to support long-term synthetic and metabolic function in what we call microfluidic, microscale cell culture analogs (CCA). The CCA concept incorporates cellular constructs each of which represents a human liver or kidney. Connecting multiple CCAs is enabled by adding to a fluid reservoir and a pump to complete the circuit. For hepatocytes, the liver CCA needs to secrete albumin and urea and maintain phase I, II & III drug toxicity functions. For kidney cells, the kidney CCA needs to excrete the drug and/or its metabolites. The combination of these two CCAs in a Liver-Kidney model allows the evaluation of not only hepatotoxicity but also drug-induced renal toxicity.
HµREL® Corporation (“Hurel”)
Technologies developed in the Center together with other technologies created at Cornell University are being advanced by Hurel, which is a privately held bioanalytic tools company with laboratories located in North Brunswick, NJ. Hurel develops and commercializes products and services based on its 3D cell-based tissue constructs (CCAs) comprised of primary human cells. It also develops its patented microfluidic devices that further enhance the already high functionality and range of application of its cell-based in vitro PBPK models. Hurel has been enabled substantially through R&D collaborations with several major pharmaceutical firms, most notably with Schering-Plough now Merck & Co.
Relevant publications
Maguire T, Usta OB, Yarmush ML. Computational fluid dynamic analysis of a cell-based microfluidic drug screening platform. NanoLIFE. 2010;1:185-194 (PMCID In Process)
Chen A, Yarmush ML, Maguire T. Physiologically based pharmacokinetic models: integration of in silico approaches with micro cell culture analogues. Curr Drug Metab. 2012 Jul;13(6):863-80. Review. PubMed PMID: 22571482; PubMed Central PMCID: PMC3966908
Bhushan A, Senutovitch N, Bale SS, McCarty WJ, Hegde M, Jindal R, et al. Towards a three-dimensional microfluidic liver platform for predicting drug. Review. PubMed PMID: 24565476;PubMed Central PMCID: PMC4028964
Hegde M, Jindal R, Bhushan A, Bale SS, McCarty WJ, Golberg I, et al. Dynamic interplay of flow and collagen stabilizes primary hepatocytes culture in a microfluidic platform. Lab Chip. 2014 Jun 21;14(12):2033-9. PubMed PMID: 24770663; PubMed Central PMCID: PMC4036071
McCarty WJ, Usta OB, Luitje M, Bale SS, Bhushan A, Hegde M, et al. A novel ultrathin collagen nanolayer assembly for 3-D microtissue engineering: Layer-by-layer collagen deposition for long-term stable microfluidic hepatocyte culture. Technology (Singap World Sci). 2014 Mar;2(1):67-74. PubMed PMID: 24932459; PubMed Central PMCID: PMC4054686
Bale SS, Golberg I, Jindal R, McCarty WJ, Luitje M, Hegde M, et al. Long-Term Coculture Strategies for Primary Hepatocytes and Liver Sinusoidal Endothelial Cells. Tissue Eng Part C Methods. 2014 Nov 6. [Epub ahead of print] PubMed PMID: 25233394
Bale SS, Vernetti L, Senutovitch N, Jindal R, Hegde M, Gough A, et al. In vitro platforms for evaluating liver toxicity. Exp Biol and Med (Maywood). 2014 Sep;239:1180-91. PubMed PMID: 24764241; PubMed Central PMCID: PMC4156546
Atienzar F, Novik E, Gerets H, Parekh A, Delatour C, Cardenas A, et al. Predictivity of dog co-culture model, primary human hepatocytes and HepG2 cells for the detection of hepatotoxic drugs in humans. Tox and Appl Pharmacol. 2014 Feb;175:44-61. PubMed PMID: 24333257
Maguire TM, Novik E, Chao P, Barminko J, Nahmias Y, Yarmush ML, et al. Design and application of microfluidic systems for in vitro pharmacokinetic evaluation of drug candidates. Curr Drug Metab. 2009 Dec;10(10):1192-9. PubMed PMID: 20166997; PubMed Central PMCID: PMC3206315
Novik E, Maguire TJ, Chao P, Cheng KC, Yarmush ML. A microfluidic hepatic coculture platform for cell-based drug metabolism studies. Biochem Pharmacol. 2010 Apr 1;79(7):1036-44. PubMed PMID:19925779; PubMed Central PMCID: PMC3136813
Kidambi S, Yarmush RS, Novik E, Chao P, Yarmush ML, Nahmias Y. Oxygen-mediated enhancement of primary hepatocyte metabolism, functional polarization, gene expression, and drug clearance. Proc Natl Acad Sci U S A. 2009 Sep 15;106(37):15714-9. PubMed PMID: 19720996; PubMed Central PMCID: PMC2747185
Chao P, Maguire T, Novik E, Cheng KC, Yarmush ML. Evaluation of a microfluidic based cell culture platform with primary human hepatocytes for the prediction of hepatic clearance in human. Biochem Pharmacol. 2009 Sep 15;78(6):625-32. PubMed PMID: 19463793
Contact
| Martin L. Yarmush, MD, PhD | Berk Usta, PhD | Rohit Jindal, PhD |
| 617-371-4882 | 617-726-3747 | 617-371-4915 |


